Arthrochalasia Ehlers-Danlos Syndrome (aEDS)
The Ehlers-Danlos syndromes (EDS) are a group of 13 related conditions that affect connective tissue (1). Connective tissue is found everywhere in the body. It connects other types of tissues, separates them, and supports them. Examples of connective tissue include bone, cartilage, fat, blood, and lymphatic tissue. When connective tissue is abnormal because of a disease, a variety of problems result.
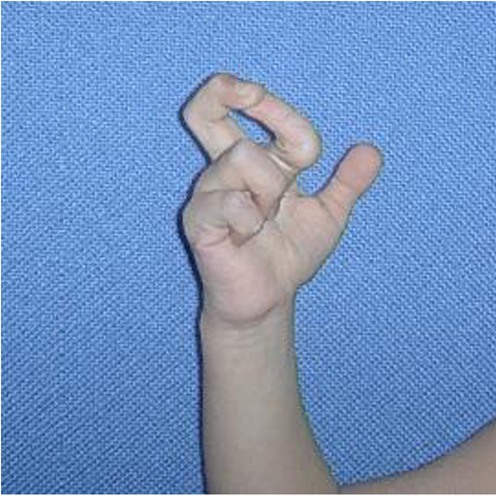
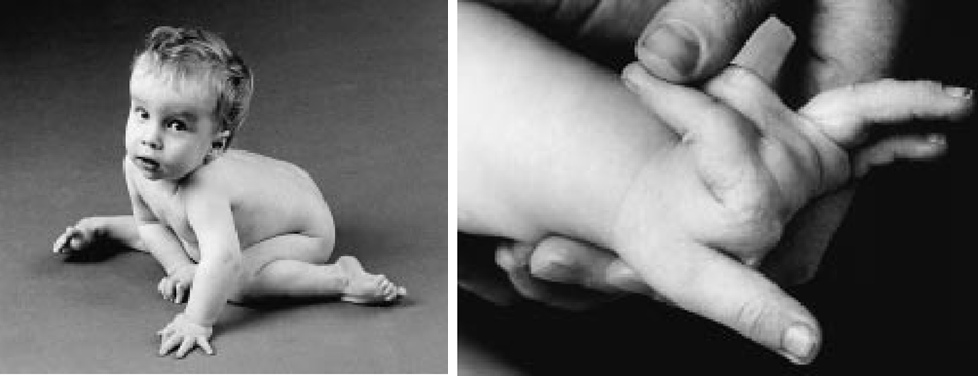
For example, certain problems happen in all of forms of EDS. The first is joint hypermobility (often called loose or double joints). People with EDS can often move their joints in ways that are impossible for most unaffected people. The photograph at the bottom of this page has an example (note that many people without EDS have loose joints, so having joint hypermobility does not mean that you have EDS). The second thing that happens in every type of EDS is stretchy (hyperextensible) skin. This feature is nearly universal in some types of EDS (such as type 1, the classical form). In our literature survey, we found that stretchy skin occured in less than half of people with the hypermobile and vascular forms, more than half of patients with the periodontitis type, and in a large majority of people with the arthrochalasia type (>80%).
Many forms of EDS happen because of mutations in genes that make collagen, which can be thought of as both holding the body together and strengthening it. Collagen gives structure to muscles, tendons, skin and bones. In most people, it is abundant in these tissues. In people with EDS, collagen may not be as strong as it should be, or there may not be enough of it. For example, skin and joints may be loose because they don't have enough collagen to hold them together securely, or because the collagen they have is weaker than it should be.
Doctors and other medical professionals assess joint hypermobility with a scoring system called the Beighton score, which was developed in the early 1970s (2). This simple system scores your ability to move a joint past certain angles. The highest score is 9 points, and higher scores mean greater joint hypermobility. Generally speaking, scores of 4 or higher (at any time, past or present) indicate joint hypermobility. Many EDS patients have scores of 8 or 9, although many have lower scores, and a minority do not have hypermobile joints. Interested readers may wish to see a basic description of the scale with a video and photos or a more technical description.
Joint hypermobility puts EDS patients at high risk for joint dislocations and partial dislocations (called subluxations). These problems can be painful, and in some cases, debilitating. Surgical procedures have been used to improve skeletal stability and improve quality of life; for examples and reviews, see references 3-5. However, experts tend to agree that non-surgical options should always be exhausted first, and careful discussions with doctors are important in this decision.
Tissue fragility is another common feature of EDS. This problem is very serious in the vascular type of EDS (vEDS). The type of collagen affected in vEDS plays a role in supporting blood vessels. When the integrity of their support system is affected, blood vessels can rupture spontaneously. This problem is especially dangerous because it affects medium and large blood vessels, as well as small ones. It also affects the bowel. Surgery on a person with vEDS must be performed with great care in order to avoid serious complications. This problem may also happen in kyphoscoliotic EDS (kEDS), but it is less common than in vEDS. Alternatively, while people with the hypermobile form (hEDS) are also prone to blood vessel ruptures, ruptures tend to occur in the small blood vessels. Tissue fragility can also lead to hernias and problems in pregnancy. Fragile skin is also a common manifestation of EDS, especially in people with the classic, kyphoscoliotic, and periodontitis types.
Tissue fragility in EDS can also lead to hernias and problems in pregnancy. Fragile skin is also a common manifestation of EDS, especially in people with the classic, kyphoscoliotic, and periodontitis types.
A new classification system for EDS was released in 2017 (1). It's called the 2017 International Classfication for the Ehlers-Danlos Syndromes. This new system names 13 different subtypes of EDS. It relies primarily on descriptive terms for different types of EDS (e.g. vascular EDS, meaning that it affects blood vessels prominently). Two other systems existed before this one. They were named Villefanche [1998] and Berlin [1988]. Both systems used names and numbers. The numbers in the new system are different from the old ones, and we've included the old ones here. Advances in genetics have allowed to researchers to find genes that cause different subtypes of EDS, which has allowed them to separate the old classic subtype (numbered 1 & 2) of EDS into 2 different subtypes.
- 1. Classical EDS (cEDS; formerly type 1/2)
- 2. Classical-like EDS (cEDS; formerly type 1/2)
- 3. Cardiac-valvular (cvEDS)
- 4. Vascular (vEDS)
- 5. Hypermobile (hEDS; formerly type 3)
- 6. Arthrochalasia (aEDS; formerly type 7a/b)
- 7. Dermatosparaxis (dEDS; formerly type 7c)
- 8. Kyphoscoliotic (kEDS; formerly type 6)
- 9. Brittle Cornea syndrome (BCS)
- 10. Spondylodysplastic (spEDS)
- 11. Musculocontractural (mcEDS)1
- 12. Myopathic (mEDS); type 12
- 13. Periodontal (pEDS; formerly type 8)
EDS affects women more commonly than men. In the literature survey we did for our analytics tool, we counted the number of male and female patients while we were characterizing in six different types of EDS. Altogether, we analyzed data from ~1,200 patients. Sex information was available for ~1,100. The table below shows that although more EDS patients are women in each type of EDS, the female:male ratio varied between each type. For example, females dominated the individuals reported with the hypermobility type, while males comprised a substantial minority of kyphoscoliotic and arthrochalasia patients. The distribution was roughly even in the periodontal type. However, the low number of case reports for these three types of EDS may have affected the outcome of this small analysis.
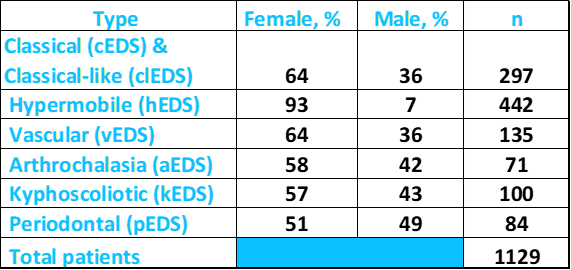
Above: Percentages of male and female patients with different types of EDS. Source: FDRF via published literature.
According to NIH's Genetics Home Reference (see link at right), the prevalence of all forms of EDS is roughly 1 in 5,000 worldwide. The kyphoscoliotic type (kEDS) is very rare, and although exact figures are not known, the GeneReviews article at the right estimates that it occurs in one birth per 100,000.
In our analysis of case histories for our analytics and diagnostic tools, we believe that we analyzed all or nearly all reported cases of the arthrochalasia subtype of EDS (70 total, with care taken not to analyze the same patient twice). The diagnosis in 62 of these patients had been confirmed by molecular or biochemical methods, with 5 of the unconfirmed cases being the well-accepted original cohort of five patients (6). Thus, the total number of reported patients for this type of EDS is higher than previous estimates of 27, 40 and 42 (54 including 12 patients from the greatest estimate (7-9).
According to NIH's Genetics Home Reference (see link at right), the prevalence of all forms of EDS is roughly 1 in 5,000 worldwide. The arthrochalasia subtype (aEDS) is very rare, and exact prevalence figures are not known.
If EDS is a condition characterized by hypermobile joints, aEDS represents the extremes of joint hypermobility in EDS. Dislocations were a universal problem in all case histories of aEDS, with congenital hip dislocations occuring in all but one patient in whom skeletal condition at birth was reported. In addition, the joints in affected individuals were typically so loose, they were reported to dislocate or sublux with minimal handling or disturbance. Surgical corrections of hip dislocations were sometimes required (10-13; list not comprehensive). The surgeries were not always successful (14), and multiple operations were sometimes required (15,16).
Clinical information
aEDS is a very rare form of EDS. Its most striking feature is recurrent dislocations, including, most notably, hip dislocations present at birth (for example, see reference 17). As noted above, all aEDS case histories to date report recurrent joint dislocations and subluxations, with a tendency for them to happen easily. These problems may be even evident duirng an ulstrasound before birth.
As with all other forms of EDS, the clinical features of aEDS overlap with other forms of EDS. The extreme joint hypermobility combined with frequent dislocations and certain facial and other features may help distinguish aEDS from other types of EDS (see Differential Diagnosis, below). In general, the following features occur commonly in this form of EDS:
- Joint hypermobility
- Recurring joint dislocations or subluxations
- Congenital hip dislocation
- Flat feet, especially when standing
- Bunions
- Clubfeet
- Abnormal curvature of the spine
- Smooth, velvety skin
- Loose or stretchy skin
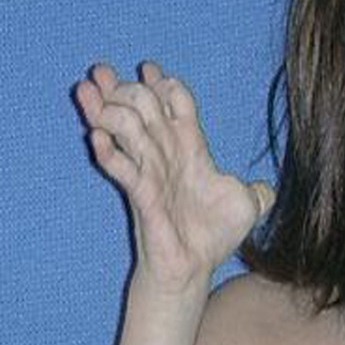
- Easy bruising (see photo at right)
- Redundant/extra skin (especially on the hands and feet; may also bunch up in folds)
- Criss-cross pattern on the palms and soles of the feet
- Hypotonia (low muscle tone; often accompanied by weakness)
- Hernia(s)
- Bone fractures in a minority of patients
Signs and symptoms of aEDS
It is possible that a criss-cross pattern on the skin, when accompanied by other signs of EDS, is suggestive of aEDS (3). Unfortunately, it is difficult to make a definite determination about this statement, given the rarity of the condition and the relatively few published case reports.
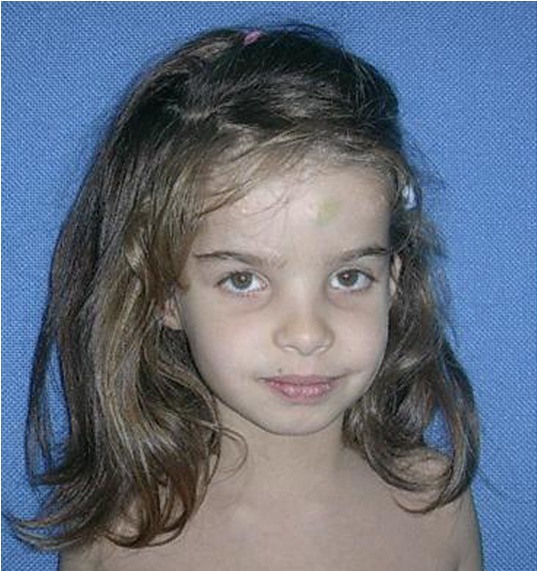
Some patients with the aEDS have distinctive facial features. These features are not as obvious, common, or numerous as they are in patients with other forms of EDS, such as vascular EDS. However, awareness of the constellation of facial features associated with each type may help with diagnosis. In aEDS, they include the following:
- Wideset eyes
- Blue sclerae
- Micrognathia (small jaw)
- Retrognathia (receding lower jaw)
- Prominent or protruding forehead (frontal bossing)
- Macrocephaly (head is large for age or body size)
Facial features that may occur in aEDS
The girl in the photograph at the top right has some of these features (wideset eyes, a prominent forehead, and a history of macrocephaly).
The arthrochalasia form of EDS comprises what were initially classified as two forms of EDS: subtypes 7A and 7B. These subtypes were named according to which gene was mutated (see below). According the literature on aEDS, the two forms are clinically indistinguishable (Giunta1999). No genotype/phenotype correlations are known at this time.
Diagnosis and Testing
Arthrochalasia EDS is an autosomal dominant disorder. This term means that it is usually passed from one affected parent to a child. However, it can also occur sporadically in a person without an affected parent. In our analysis, information on the family history of EDS or EDS-like connective tissue disease was available for 50 patients. Of this group, only 17, or 34%, had an affected parent.
Mutations in one of two collagen genes can cause aEDS; the genes are COL1A1 and COL1A2. These genes encode two proteins that combine to form type I collagen. Each gene forms a different chain on the final protein. Type I collagen is the most abundant collagen in humans. It is found in scar tissue, which explains why some aEDS patients have abnormal scars and problems with wound healing. It also occurs in bones, tendons, and ligaments. Ligaments connect bone to bone and have an important role in maintaining joint stability. In aEDS, mutations in either gene generally occur in exon 6 (see Differential Diagnosis below for more information on the importance of this point).
In our analysis, mutation was known in 62 people. COL1A1 was mutated in 10 of them (~26%), while 46 (74%) had mutations in COL1A2.
In addition, mutations in the gene COL1A1 account for a small minority of cases of classic EDS. Mutations in COL1A1 are associated with a number of other medical conditions, including osteogenesis imperfecta and Caffey disease. Mutations in COL1A2 are also associated with osteogenesis imperfecta, as well as with the cardiac valvular form of EDS. This latter condition is rarer than aEDS and is an autosomal recessive disorder (both parents must pass a copy of the mutated gene to their child).
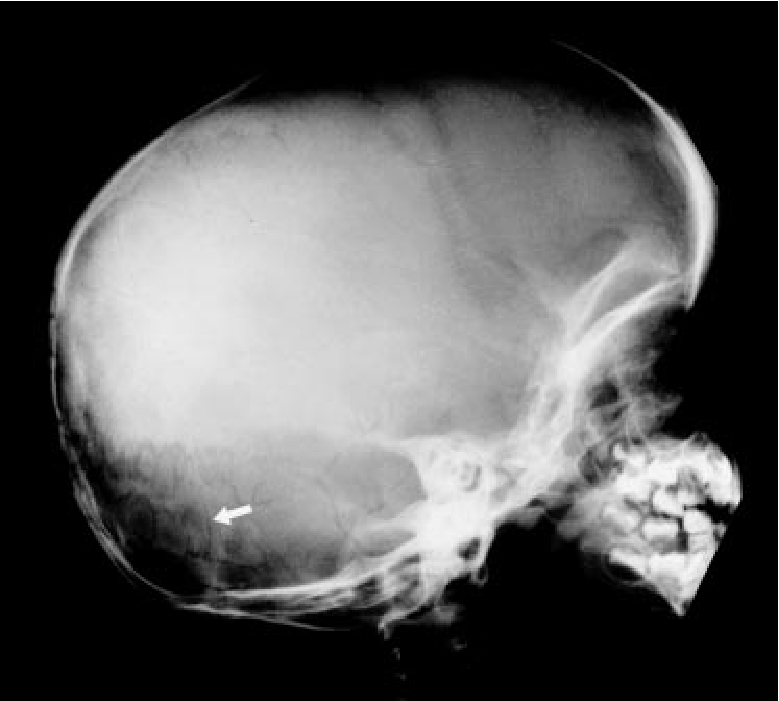
The initial diagnosis of aEDS is based on the clinical features of hip dislocation (especially if present at birth), hypotonia, and some or all of the facial features noted above. A criss-cross pattern on the palms and/or soles of the feet is also suggestive of this disorder. Patients may also have Wormian bones (see x-rays and photos below). This abnormality has been reported in nine patients out of ten surveyed, and although the sample size is small, further investigation may reveal that it is a common feature of aEDS.
Laboratory testing may help confirm a diagnosis, with the caveats noted under Differential Diagnosis below. There are two general methods of testing. The first is to characterize collagen I aminoterminal propeptides in cultured fibroblasts. The second is to perform sequence analysis of exon 6 in the COL1A1 or COL1A2 genes. The second method may be less invasive.
It is important to remember that there is a lot of overlap between the different forms of EDS and between aEDS and osteogenesis imperfecta. This fact means that, in the absence of laboratory testing, accurate subcategorization of EDS in a patient may be difficult or even impossible.
Differential Diagnosis
Osteogenesis imperfecta (OI) can also be caused by mutations in type 1 collagen. The clinical features of OI vary. The severe forms are progressive and degenerative, and may involve underdeveloped rib cages and lungs, and early lethality. They are unlikely to be confused with aEDS. However, the mild forms overlap with aEDS. An example is OI type 1, which is now called classic non-deforming OI with blue sclerae. People with this type of OI suffer bone fractures as a result of minor or no trauma (e.g. diapering an infant). Fractures can occur in the newborn period or early infancy, but more usually tend appear when a child begins to walk (and fall). They continue through adolescence and then tend to abate. However, they may begin to occur again after menopause in women or after age 40 in men. In addition, OI patients can have lax joints, low muscle, and skin that bruises easily. As the name of the condition says, the whites of a patient's eyes are frequently blue or grey-ish. This is also the case with many aEDS patients. Height and teeth may be affected in both OI and aEDS. Thus, the two disorders are very similar.
Mild OA can be caused by mutations in COL1A1 or COL1A2. Given that mutations in the same gene are involved in both conditions, it is possible that aEDS is a mild form of OI. For example, in a literature review using the OI mutations database (18), we found a patient with mutations in exon 6 of COL1A1 who had diagnosed with OI type 1. In addition, researchers have also found patients with mutations in COL1A1 and a disorder described as combined OI/EDS7 (19).
Other forms of EDS. The differential diagnosis for aEDS also includes all the other types of EDS. All forms of EDS involve joint hypermobility, and most also feature very soft, lax skin, as well as a variety of internal problems that result from weak or loose connective tissue (e.g. hernias, spinal column deformities, flat feet). However, some forms of EDS are more severe than others.
aEDS may be distinguished from the rare vascular and kyphoscoliotic types by genetic testing: the vascular form is caused by mutations in COL3A1, and the kyphoscoliotic form is caused by mutations in PLOD1 or FKBP14. In addition, these conditions are generally (although not always) much more severe than the arthrochalasic form. Patients with vascular EDS have a particular facial appearance that isn't shared by classical EDS patients, and those with the kyphoscoliotic form tend to have severe spinal deformities. aEDS patients may also have spinal deformities, however. Note also that EDS-vascular patients are prone to spontaneous ruptures of large blood vessels that may be fatal. For this reason, clinicians may wish to use genetic testing to definitively rule out this form of EDS in any patient suspected of having EDS.
The classical form of EDS (cEDS) ranges in severity from mild to relatively severe. Like aEDS patients, classical EDS patients have hypermobile joints and stretchy skin. Some features may help distinguish the two disorders. Most importantly, cEDS patients tend to have severe skin involvement that can be disfiguring, while aEDS patients do not. Alternatively, cEDS patients do not tend to suffer the serious and recurrent joint dislocations that are universal in aEDS. Dislocations do occur in people with cEDS (roughly half of patients, according to our analysis). However, the constancy of the problem in aEDS and the presence of congenital hip dislocations may be a distinguishing factor.
The hypermobile form of EDS (EDS-H) is a relatively mild form of EDS. As its name implies, joint hypermobility is the primary feature of this form of EDS. The skin in EDS-H patients is typically not as stretchy as it is in patients with the other forms of EDS, although exceptions apply. Atrophic scars also do not appear to be a general feature of the hypermobile form of EDS-H. They appear to occur in roughly half of aEDS patients.
Overall, joint hypermobility occurs in a variety of medical conditions, and many people without a medical condition have lax joints. In fact, when not part of a disorder, hypermobile joints confer an advantage in sports such as gymnastics and ice skating, as well as in certain forms of dance.
Marfan syndrome. Most medical conditions involvong lax joints are relatively easy to distinguish from EDS, but some are not. Marfan syndrome is an example. Like EDS, Marfan syndrome is a connective tissue disorder and patients have lax joints. Marfan patients generally have a body type called a Marfanoid body habitus. This term means that a person is tall and slender, with long arms, hands, fingers, legs, feet and toes. A Marfanoid body habitus occurs in a minority of EDS-H patients, but it does not appear to be generally associated with classic EDS. The Marfan Foundation has many photographs of Marfan patients of different ethnicities. Unlike EDS patients, Marfan patients do not appear to have fragile skin and blood vessels.
Cutis laxa. Superficially, the stretchy skin found in most forms of EDS may be confused with cutis laxa type 1 and type 2, as stretchy skin is also a feature of these conditions. However, the skin in cutis laxa patients tends to sag and does not return to its normal position after being extended. Cutis Laxa patients often have a prematurely aged appearance.
References
- 1. Malfait F et al. (2017) The 2017 International Classification of the Ehlers-Danlos Syndromes Am J Med Genet C 175(1):8-26. Abstract on PubMed.
- 2. Beighton P et al. (1973) Articular mobility in an African population. Ann Rheum Dis 32(5):413-418. Full text on PubMed.
- 3. Cole AS et al. (2000) Recurrent instability of the elbow in the Ehlers-Danlos syndrome. A report of three cases and a new technique of surgical stabilisation. J Bone Joint Surg Br 82(5):702-704. Full text from publisher.
- 4. Weinberg J et al. (1999) Joint surgery in Ehlers-Danlos patients: results of a survey. Am J Orthop 28(7):406-409. Abstract on PubMed.
- 5. Shirley ED et al. (2012) Ehlers-Danlos syndrome in orthopaedics. Etiology, diagnosis, and treatment implications. Sports Health 4(5):394-403. Full text on PubMed.
- 6. Hass J & Hass R (1958) Arthrochalasis multiplex congenita; congenital flaccidity of the joints. J Bone Joint Surg Am 40-A(3):663-674. Abstract available through publisher.
- 7. Klaassens M et al. (2012) Ehlers-Danlos arthrochalasia type (VIIA-B)--expanding the phenotype: from prenatal life through adulthood. Clin Genet 82(2):121-130. Full text on PubMed.
- 8. Melis D et al. (2012) Cardiac valve disease: an unreported feature in Ehlers Danlos syndrome arthrocalasia type? Ital J Pediatr 38:65. doi: 10.1186/1824-7288-38-65. Full text on PubMed.
- 9. Ayoub F et al. (2017) Clinical features, molecular results, and management of 12 individuals with the rare arthrochalasia ehlers-danlos syndrome Am J Med Genet A 182(5):994-1007. Abstract on PubMed.
- 10. Giunta C et al. (1999) Ehlers-Danlos syndrome type VII: Clinical features and molecular defects. J Bone Joint Surg Am 81(2):225-238. Abstract on PubMed.
- 11. Giunta C et al. (2008) The arthrochalasia type of Ehlers-Danlos syndrome (EDS VIIA and VIIB): the diagnostic value of collagen fibril ultrastructure. Am J Med Genet A 146A(10):1341-1346. Abstract on PubMed.
- 12. D'Alessio M et al. (1991) Characterization of a COL1A1 splicing defect in a case of Ehlers-Danlos syndrome type VII: further evidence of molecular homogeneity. Am J Hum Genet 49(2):400-406. Full text on PubMed.
- 13. Cole WG et al. (1987) The clinical features of Ehlers-Danlos syndrome type VII due to a deletion of 24 amino acids from the pro alpha 1(I) chain of type I procollagen. J Med Genet 24(11):698-701. Full text on PubMed.
- 14. Byers PH et al. (1997) Ehlers-Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagens. Am J Med Genet 72(1):94-105. Abstract on PubMed. Full text from authors.
- 15. Vasan NS et al. (1991) A mutation in the pro alpha 2(I) gene (COL1A2) for type I procollagen in Ehlers-Danlos syndrome type VII: evidence suggesting that skipping of exon 6 in RNA splicing may be a common cause of the phenotype. Am J Hum Genet 48(2):305-317. Full text on PubMed.
- 16. Wirtz MK et al. (1987) Ehlers-Danlos syndrome type VIIB. Deletion of 18 amino acids comprising the N-telopeptide region of a pro-alpha 2(I) chain. J Biol Chem 262(34):16376-16385. Full text from publisher.
- 17. Carr AJ et al. (1994) The clinical features of Ehlers-Danlos syndrome type VIIB resulting from a base substitution at the splice acceptor site of intron 5 of the COL1A2 gene. J Med Genet 31(4):306-311. Full text on PubMed.
- 18. Osteogenesis Imperfecta Variant Database
- 19. Cabral WA et al. (2005) Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Anat Physiol 280(19):19259-19269. Full text from publisher.
- 20. Barclay-Smith E (1909) A rare condition of Wormian ossifications. J Anat Physiol 43(Pt 3):277-278. Full text on PubMed.
- 21. Sobotta J. (1914) Atlas and text-book of human anatomy. Philadelphia: W. B. Saunders Company.