Beckwith-Wiedemann Syndrome (BWS)
Beckwith-Wiedemann Syndrome (BWS) is a member of a group of conditions called overgrowth syndromes. Although individual overgrowth syndromes are often very different from one another, they all involve overgrowth of the whole body (e.g. excessive length and weight at birth) or a part of the body (e.g. large abdominal organs or a large tongue). Other members of this group include Sotos syndrome, Pallister-Killian syndrome, Proteus syndrome, neurofibromatosis type I (NF1), Weaver syndrome, Simpson-Golabi-Behmel syndrome, Sturge-Weber syndrome, macrocephaly-capillary malformation, and fragile X syndrome. Fragile X syndrome and NF1 are the most common members of this group.
Clinical information
BWS is a rare genetic disease characterized by pediatric gigantism and characteristic abnormalities of fetal development. It is estimated to occur in 1 in 13,700 births, though this may be an underestimate as milder cases may be unrecognized (1). BWS occurs in all ethnic groups and in both genders. Assistive reproductive technology (ART) is also associated with an increased incidence of BWS (1-3). The syndrome may be somewhat more common in identical twins, but expression is usually variable between them (2, 4). When observed in identical twins, the majority are female (2, 4).
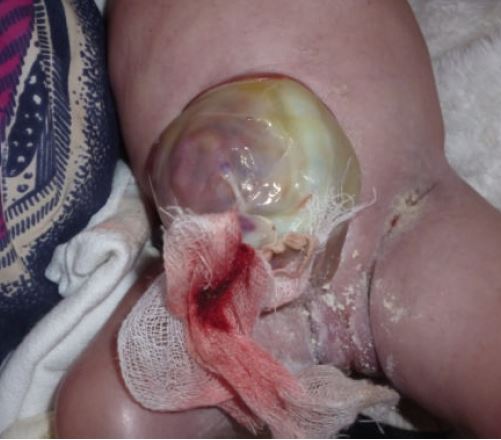
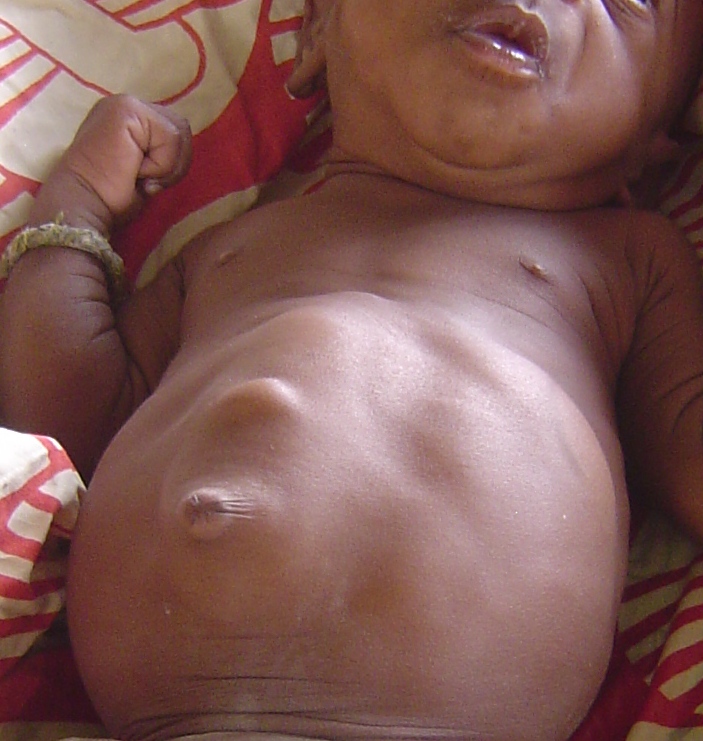
BWS is most easily recognized by a constellation of features that are present at birth and during the first several years of life (2). Premature infants with BWS may be large for gestational age (length and weight). In some newborns, intestines or other abdominal organs protrude through the navel (see photo at right). This condition is called omphalocele or exomphalos. It results from rapid fetal growth and a failure of the abdominal wall to fully close during development. Umbilical hernias and other abdominal wall abnormalities also occur (see photos below and at right; 5).
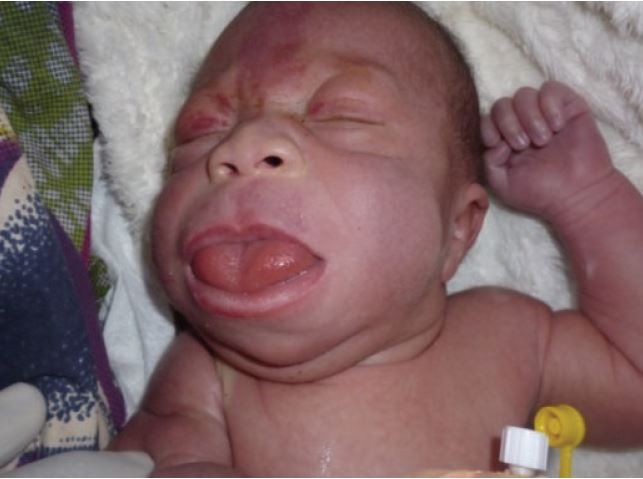
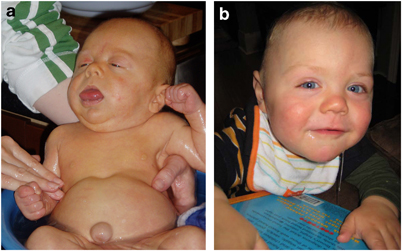
Certain facial features are typical of BWS (see photos at right and below), and are most obvious before the age of 3 years. The most obvious of the facial features is an enlarged tongue macroglossia). Mild or moderate macroglossia may improve due to growth of the head and mouth during early childhood. However, when severe, the problem may require surgery. However, in many cases, head and mouth growth After age five, facial features tend to be subtle (1). Macroglossia is generally not apparent after age 8.
Clinical features that may occur in BWS include the following:
- Macroglossia (enlarged tongue, may protrude from mouth)
- Large size for gestational age
- Abdominal abnormalities (organ enlargement, hernias, distention of abdomen)
- Nevus flammeus (red skin on forehead and/or eyelids) especially in infancy
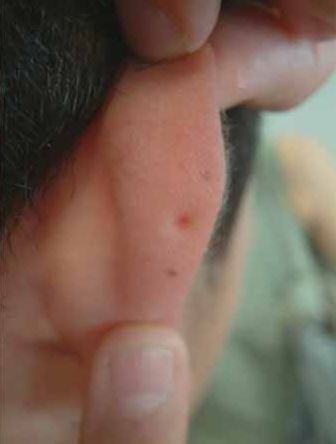
- Ears; pits, dimples, or creases on external ear
- Hypoglycemia
- Hemihypertrophy, also called hemihyperplasia (one side of the body is larger than the other)
- Midface hypoplasia (undervelopment of the face between the upper jawa and eyes)
- Undescended testes in boys
- Abdominal tumors
- Advanced bone age
Common problems in BWS
A number of other problems may also occur in BWS. Other features include heart disease (such as cardiomyopathy), cleft palate, polyhydramnios (extra amniotic fluid during pregnancy) and premature birth.
Fetal and childhood tumors are common in BWS. Around 20% of patients will develop one or more tumors during infancy or childhood; adrenocortical carcinoma, hepatoblastoma, neuroblastoma, rhabdomyosarcoma, and Wilms' tumor all occur in BWS (1-3; 5).
BWS itself does not generally cause intellectual disabilities, although certain paternal chromosomal duplications are associated with a high risk of this problem (1). However, many people with BWS suffer neurological damage due to uncontrolled hypoglycemia during the neonatal period. This problem can cause cognitive disabilities. However, if blood glucose is controlled in infant patients, damage can be avoided (3). Prenatal diagnosis allows for clinicians and caregivers to anticipate these problems and helps them prepare to meet the needs of infants with BWS (1-3; 5).
Diagnosis and Testing
There are no absolute requirements for a diagnosis of BWS. However, there are major and minor findings associated with BWS that helpful for diagnosis (1, 3). A diagnosis of BWS is supported if a child has 3 major findings or 2 major finding and 1 minor finding as outlined in the table below:
- Defect in abdominal wall (omphalocele, umbilical hernia)
- Macroglossia
- Large size (height/weight >97th percentile)
- Ears; pits, dimples, or creases on external ears
- Abdominal enlargement/visceromegaly
- Embryonal tumor during childhood
- Hemihypertrophy/hemihyperplasia (one side of the body is larger than the other)
- Kidney abnormalities such as medullary dysplasia and development of medullary sponge kidney
- Family history of BWS
- Cleft palate
- Abnormalities during pregnancy, including polyhydramnios, enlarged placenta and/or thickened umbilical cord; and prematurity
- Hypoglycemia in the neonatal period
- Nevus flammeus (red skin on forehead and/or eyelids) especially in infancyCardiac abnormalities, including enlarged heart, structural anomalies, and/or cardiomyopathy
- Characteristic facial appearance
- Diastastis recti
- Advanced bone age
A newborn or fetus who is suspected of having BWS should undergo a thorough clinical examination followed by possible molecular genetic investigation. Several genetic syndromes have features in common with BWS. The link in the right-side column of this page will take you to information about labs that test for BWS.
BWS can be caused by abnormalities in the way that genes in a portion of chromosome 11 are regulated. In this region, some genes that come from a person's mother are turned off, and some genes that come from a person's father are turned off. This process is called genomic imprinting. When the usual pattern of imprinting changes, a syndrome can result. Imprinting problems account for roughly half of all cases of BWS. In a further 20% of cases, a person has two copies of paternal genes in this region of chromosome 11, rather than one copy from their mother and one from their father. People with this form of BWS tend to have intellectual disabilities more often than people with other forms of BWS.
Other problems that can lead to BWS include mutations in the gene CDKN1C, which is involved in the control of growth before birth. CDKN1C is located in the same region of chromosome 11 that was noted in the last paragraph. Several other problems can also lead to BWS in a very small number of cases (around 1%). They include rearrangements, duplications, and losses of genetic material in the same region of chromosome 11.
Differential diagnosis
Simpson-Golabi-Behmel syndrome (SGBS). SGBS is caused by a mutation in the gene GPC3, which controls growth. SGBS is an X-linked recessive condition. This term means that it is passed from mothers to sons via the X chromosome. However, some signs of disease may be seen in female carriers. SGBS shares many features with BWS (e.g. overgrowth, umbilical hernia, posterior helical ear pits). It can be misdiagnosed as BWS (6). The two conditions can be distinguished via macroglossia, body asymmetry, and ompholocele, which typify BWS. In SGBS, fetal overgrowth is almost universal. In BWS, fetal growth may be nearly normal until the antenatal period. Finally, SGBS patients also frequently have post-axial polydactyly, an uncommon finding in BWS.
Most cases of BWS are sporadic, meaning that they happen apparently randomly, without an identifiable reason. In this regard, a careful family history may also be useful for distinguishing BWS and SGBS, because half the male children of a female carrier will be affected (Vora). Prenatal testing is available if there is a known mutation in the family.
Perlman syndrome (PS). The clinical features of Perlman syndrome are simmilar to those of BWS, but often more severe. PS also has a high perinatal mortality (64%; 7). Its pathogenesis is still not entirely understood, but it seems to be inherited in a recessive pattern (3, 7). This term means that both parents must pass a copy of a mutated gene to a child. Typical clinical features of Perlman syndrome include bilateral (symmetric) renal enlargement with fetal (not neonatal) macrosomia, hydronephrosis, hydroureter, cryptorchidism, and developmental delay (7). Characteristic facial features include a small upturned nose with a deep crease at the nasal bridge, and may have a small mouth (7). These facial features are not usually observed in BWS. While cryptorchidism and abnormalities of the genitourinary organs are common to both syndromes, developmental delays are unusual in BWS patients whose airways and blood glucose have been carefully managed (3, 7). Parents of children diagnosed with Perlman syndrome should be avised that there is a 25% risk that future children will be affected (7).
Sotos syndrome is caused by mutations in the gene NSD1. Most cases of Sotos syndrome are sporadic, although autosomal dominant inheritance from a parent has been reported (7). This term means that the disease can be passed to a child by a single parent with a mutated gene. Sotos syndrome is typified by pre- and post-natal overgrowth and advanced bone age, problems that overlap with BWS. Prenatally, the child may have a distinctive Sotos-like appearance, but this is not always the case, and the appearance may be similar to BWS. Unlike BWS, however, people with Sotos syndrome tend to have developmental delays, macrocephaly (large heads) or dolichocephaly (a long, narrow skull), possible frontal bossing of the forehead, and large hands and feet. Also unlike patients with BWS, all patients with Sotos syndrome have brain abnormalities via MRI (7). Not all fetuses demonstrate early overgrowth in Sotos syndrome, however.
All four syndromes noted here should be considered in the differential diagnosis of overgrowth with enlarged abdominal organs. Distinction between the syndromes can sometimes be made via genetic testing, or by careful evaluation of the unique features of each characteristic phenotype during infancy, as noted above. The tumors which can present in BWS may be a distinguishing feature in older patients (1-3). Additionally, most cases of BWS can be distinguished via macroglossia, hemihyperplasia (body asymmetry), and omphalocele that typify BWS and are relatively unusual in the other diagnostic possibilities.
Treatment and Monitoring
The management of BWS typically involves standard supportive medical and surgical strategies. It is helpful to consider the complications of BWS by the age at presentation (1, 3). If there are prenatal findings suggesting BWS, it is important to be prepared to manage airway compromise due to macroglossia. Screening for hypoglycemia should be undertaken in the first few days of life, and caregivers should be advised that it may occur later in the neonatal period. Tumor surveillance, via abdominal sonography several times annually, is also very important (Vora). This is particularly crucial if BWS derived from methylation changes at imprinting center two is the confirmed molecular diagnosis (1). After childhood, complications due to BWS are thought to be rare (2).
Genetics
BWS is caused by errors in expression and regulation of the imprinted genes of chromosome 11p15.5. Imprinting is the epigenetic control of duplicate genes so that one inherited copy is silenced, while the other is expressed (1,3). Dysregulation of imprinted genes in the 11p15.5 imprinted region results in the BWS phenotype through a number of different mechanisms leading to either primary epigenetic or genetic changes that modify the relative contributions of alleles inherited from either parent. This chromosomal region includes two functional domains, each governing different genes related to growth; paternally expressed genes have growth enhancing activities and the maternally expressed genes (paternally imprinted) have growth suppressing activity (3). Imprinting center one regulates production of insulin-like growth factor (IGF2), a paternally-expressed fetal growth factor. A gain of methylation at the domain 1 imprinting center is found in about 5% of patients and leads to overexpression of IGF2 since neither gene copy is silenced, which can cause macrosomia (fetal overgrowth). The second imprinting region regulates several genes, including KCNQ1, KCNQ1OT1, and CDKN1C, which regulates cell proliferation. About 50% of BWS patients have a loss of methylation resulting in dysregulation at imprinting center two, leading to reduced expression of CDKN1C. This particular defect can lead to a higher probability of childhood tumors (1). Loss of maternal methylation at imprinting center two is the epigenetic defect most commonly observed in BWS cases in pregnancies which are the result of ART (1, 4). In others instances, there may be microdeletions or other forms of dysregulation involving one or both imprinting centers, or another mechanism such as inheriting two copies of the chromosome region from one parent (paternal uniparental disomy, UPD; 3). Current studies of BWS patients identify a chromosome 11p15 abnormality in less than 80% of individuals with a BWS phenotype (1, 3, 8).
Most cases of BWS occur spontaneously (with no family history of the disorder), and are not likely to recur in a family. There are some cases in which a dominant inheritance pattern exists, usually of CDKN1C mutation. Genetic testing is required to rule out a heritable defect, which can lead to 50% risk of passing the disease to an affected child's future siblings (1, 3). Particular genetic mechanisms lead to different phenotypes among BWS patients; testing to determine the underlying defect may be quite important to management and surveillance throughout childhood. This is particularly true of tumor surveillance. For a more thorough treatment of the variety of genetic mechanisms underlying BWS, please see references 1 and 3.
References
- 1. Weksberg R et al. (2010) Beckwith-Wiedemann syndrome. Eur J Hum Gen 18(1): 8-14. Full text on PubMed.
- 2. Elliott M & Maher ER (1994) Beckwith-Wiedemann syndrome Med Genet 31:560-564. Full text on PubMed.
- 3. Gicquel C et al (2005) Beckwith-Wiedemann syndrome. Orphanet encyclopedia. Full text.
- 4. Weksberg R et al. (2005) Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet. 137C(1):12-23. Abstract on PubMed.
- 5. Prescott T & Hennekam R (2007) Posterior helical pits. Eur J Med Genet 50(2):159-161. Abstract on PubMed.
- 6. Elliott M et al. (1994) Clinical features and natural history of Beckwith-Wiedemann syndrome: presentation of 74 new cases. Clin Genet 46(2): 168-174. Abstract on PubMed.
- 7. Vora N & Bianchi D (2009) Genetic considerations in the prenatal diagnosis of overgrowth syndromes. Prenat Diagn 29(10):923-929. Full text on PubMed.
- 8. Engström W et al. (1988) Wiedemann-Beckwith syndrome. Eur J Pediatr 147: 450-457. Abstract on PubMed.
- 9. Mbuyi-Musanzayi S et al. (2014) Meningocele in a Congolese Female with Beckwith-Wiedemann phenotype. Case Rep Genet 2014:989425. Full text on PubMed.
- 10. Photo of an infant with diastastis recti. Photo by Wikigil and found on the Wikimedia Commons.
- 11. National Human Genome Research Institute. Elements of Morphology: Human Malformation Terminology (Online encyclopedia).